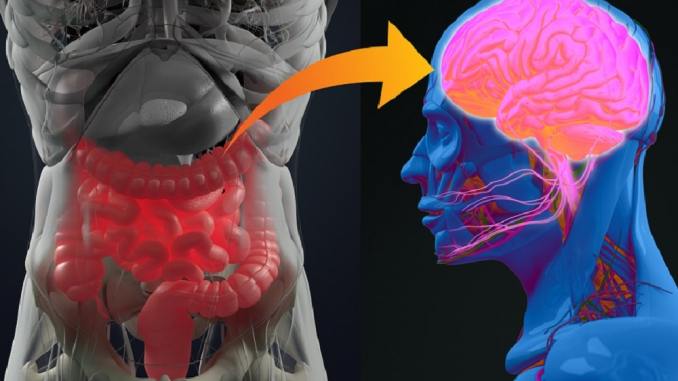
Representing more than 99% of our genetic material, the friendly microbes in our bodies take up a lot of space. Mainly residing in the gut, our bugs were here first, have co-evolved with us, and continue to play vital roles in both our early development and lifelong health. This article briefly discusses those roles over time, with particular emphasis on gut-brain pathways. Also, simple lifestyle considerations to help improve stress management and mental well-being by properly caring for our commensal community, sometimes referred to as our second brain, is also reviewed.
Early Life
An infant’s gut environment, or microbiome, is initially “seeded” at the time of birth. How we arrive – by vaginal delivery or C-Section – determines the initial microbial composition (species and strains that begin to inhabit the gut). These resident bugs not only influence the developing infant microbiome, but they also impact immune and nervous system development.[1],[2] Interestingly, exposure to the microbes during vaginal birth tends to be associated with better health over the long term. Whereas C-Section babies, who are initially exposed to microbes present on the mother’s skin, run a higher risk of developing allergy, autoimmune disease, and mood disorders.[3] Fortunately, supportive measures like avoiding antibiotic use, breastfeeding, and even having pets in the home can bolster the microbiome and help mitigate those risks.
The Turbulent Teens
As any parent can attest, the adolescent brain undergoes many developmental and hormonal changes in the teen years. Although sex hormones increase dramatically in puberty, stress hormones tend to remain relatively constant. On the other hand, environmental stressors tend to lead to exaggerated stress hormone levels during these years. Such persistent stress response has also been associated with increased and prolonged anxiety and depressive behavior.[4] Data further emphasizes the influence of key gut-brain communications pathways, the bi-directional hypothalamic-pituitary-adrenal (HPA) axis in particular, which is itself undergoing development during these years. It is understandable therefore that continual, unmitigated stress during this period can result in an imbalance, negatively impacting cognition, decision making, and mood over the long term.[5]
 |
The Gut-Brain Axis Microbes in our guts communicate with our brains in a continuous, bidirectional manner through the HPA axis, the vagus nerve, and systemic blood circulation.• Gut microbes communicate via chemical networking amongst each other (quorum sensing) and nearby immune and intestinal cells to help balance immune response and intestinal barrier integrity.• The bidirectional, neuroendocrine hypothalamic-pituitary-adrenal (HPA) axis connects the brain with the gut to help manage stress induced cortisol production. Notably, exaggerated stress response has been linked directly to microbiome imbalance.• The longest nerve in the body, the vagus, uses sensory and motor nerves to link our brains to our guts. Although bidirectional in nature, most of the information flows from the gut to the brain in the form of neurotransmitters and inflammation regulating proteins. |
Maturity
Although unique to each individual, the makeup of our gut microbiome becomes more stable as we enter adulthood.[6] Microbe diversity is however reduced over time and in the face of illness and medications, especially antibiotics. In fact, it has been suggested that age-dependent changes in microbiome diversity may influence human frailty, immune function, and disease development[7] ranging from depression[8] to Parkinson’s.[9] Inflammation is significant contributor to these conditions, resulting from, among other factors: the leakage of toxic microbial metabolites through an overly permeable intestinal barrier (a.k.a. leaky gut), and an imbalance of inflammation modulating proteins. The resulting systemic inflammation (metaflammation) is also sometimes described as “inflamm-aging”[10] owing to its direct association with age-related decline.
Another key area of interest lies in the brain’s immune cells.[11] Acting as scavengers within the central nervous system, specialized microglia cells rely on the support of our gut microbes to mature, develop, and function. Without healthy microglia, we are literally unable to effectively clear the accumulating debris from our brains. Also of note is the fact that our microbes are directly involved in the production of all five mood altering neurotransmitters: GABA, serotonin, acetylcholine, norepinephrine, and dopamine. Surprisingly, the vast majority of serotonin is, in fact, produced in the gut.[12]
Right Sizing Inflammation
I have long referred to the antecedents of metaflammation in an acronym: GULCH. Whereas genes, uterine environment, life experience, choices, and habits all play instrumental roles in predisposition to inflammatory diseases including depression, irritable bowel, arthritis, obesity, and a variety of immune related conditions.
| Metaflammation Pathways
Microbes in our guts communicate with our brains in a continuous, bidirectional manner through the HPA axis, the vagus nerve, and systemic blood circulation.
|
 |
Despite the aspects beyond our control, we are certainly able to effectively manage many of the triggers that lead to uncontrolled metaflammation. Simple lifestyle modifications include:
- Effective management of existing medical
- Excess weight loss
- Colorful, whole food diet rich in prebiotic fibers and lean protein
- Eight hours of quality sleep every night
- Regular cardiovascular and weight bearing exercise
- Smoking Cessation
- Alcohol and sugar moderation
- Friendship and gratitude
 |
 |
The Promise of Psychobiotics
If [gut] microbes are controlling the brain, then microbes are controlling everything.
John Cryan, PhD APC Microbiome Institute
Another tool in the arsenal is a special class of probiotics targeted to support the gut-brain axis. First coined by the team of John Cryan, PhD and Ted Dinan, MD, PhD at the Alimentary Pharmabiotic Center at University College, Cork, Ireland, the term psychobiotics refers to beneficial bacteria (probiotics) or support for such bacteria (prebiotics) that influence bacteria–brain relationships.
Well-characterized psychobiotics can help manage stress, anxiety, and mood through right sizing inflammatory response as well as hormonal/HPA balance. But not all probiotic strains are psychobiotic, and not all probiotic blends claiming psychobiotic characteristics are backed by scientific evidence. Therefore, when seeking a probiotic that will effectively support the gut-brain axis, it is important to understand how the formulation was developed and what clinical evidence exists to demonstrate its efficacy.
-
Strain Significance
Akin to dog breeds, various microbial (probiotic) strains within a species do not possess the same set of characteristics. It is therefore important to understand the mechanistic significance of the individual strains in any given formulation. Fortunately, probiotic manufacturers have a multitude of tools that allow for the identification of each. From transepithelial electrical resistance testing to assess the ability to strengthen the intestinal barrier, to quantifying the production of anti-inflammatory proteins and neurotransmitters, selecting the right characteristic for the desired outcome is the first step.
-
Formulation Synergy
At least as important as individual strain functionality is the complementary and collaborative effects of a highly effective psychobiotic formulation. Although certainly hypothesized in the formulation development process, the only reliable method to determine if the probiotic blend is functioning as it should is through controlled clinical trials. Such trials are complicated and expensive, but they also provide a much higher level of confidence that the formulation will work as expected.
-
Microbial Survival
The last consideration in selecting an effective psychobiotic is assurance that the formulation provides a robust environment for the probiotic microbes to endure the gastric environment and arrive in the intestines with full metabolic activity. Fortunately, modern techniques make it a simple matter for manufacturers to test survival and stability.
Alternative and integrative healthcare practitioners can be great resources for selecting an effective probiotic formulation. But the quality process as described in the 3-S steps above (Significance, Synergy, Survival) should also be readily available from the probiotic manufacturer—and taking the simple steps to investigate the data can make the difference between effective psychobiotic support and quite literally, flushing money down the toilet.
Stay tuned for upcoming articles in this series which will dive more deeply into how our microbiomes are related to specific neurological conditions including depression/anxiety, Alzheimer’s and Parkinson’s and what steps you can take to better support your gut-brain axis.
[1] Moya-Pérez A, Luczynski P, Renes IB, et al. Intervention strategies for cesarean section-induced alterations in the microbiota-gut-brain axis. Nutr Rev. 2017;75(4):225-240. doi:10.1093/nutrit/nuw069
[2] Nash MJ., et al. Early Microbes Modify Immune System Development and Metabolic Homeostasis—The “Restaurant” Hypothesis Revisited, Front Endocrin. 2017;8:349
[3] Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol. 2011;38(2):321-331. doi:10.1016/j.clp.2011.03.008
[4] McVey Neufeld KA, Luczynski P, Dinan TG, Cryan JF. Reframing the Teenage Wasteland: Adolescent Microbiota-Gut-Brain Axis. Can J Psychiatry. 2016;61(4):214-221. doi:10.1177/0706743716635536
[5] McEwen, B. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress. 2017 Jan-Dec; 1: 2470547017692328.
[6] O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015 Dec 4;350(6265):1214-5. doi: 10.1126/science.aac8469. PMID: 26785481.
[7] Galkin F, Aliper A, Putin E, Kuznetsov I, Gladyshev VN, Zhavoronkov A. Human microbiome aging clocks based on deep learning and tandem of permutation feature importance and accumulated local effects. bioRxiv. 2019. 10.1101/507780.
[8] Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front Psychiatry. 2020;11:541. Published 2020 Jun 10. doi:10.3389/fpsyt.2020.00541
[9] Wallen ZD, Appah M, Dean MN, et al. Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. NPJ Parkinsons Dis. 2020;6:11. Published 2020 Jun 12. doi:10.1038/s41531-020-0112-6
[10] Kim KA, Jeong JJ, Yoo SY, Kim DH. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016;16:9 Epub 2016/01/17. 10.1186/s12866-016-0625-7
[11] Jyothi HJ, Vidyadhara DJ, Mahadevan A, Philip M, Parmar SK, Manohari SG, Shankar SK, Raju TR, Alladi PA. Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol Aging. 2015 Dec;36(12):3321-3333. doi: 10.1016/j.neurobiolaging.2015.08.024. Epub 2015 Aug 31. PMID: 26433682.
[12] Wall R., Cryan J.F., Ross R.P., Fitzgerald G.F., Dinan T.G., Stanton C. (2014) Bacterial Neuroactive Compounds Produced by Psychobiotics. In: Lyte M., Cryan J. (eds) Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease. Advances in Experimental Medicine and Biology, vol 817. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0897-4_10


Leave a Reply